What is tissue-agnostic therapy?
The rise of tissue-agnostic therapies is encouraging more providers to use biomarker testing to guide diagnosis and treatment selection. Biomarker testing essentially provides a genetic map of what is driving an individual’s cancer. Providers can use this map to pinpoint which treatment is likely to offer the best response.

A paradigm shift in oncology
In May 2024, Dr. Razelle Kurzrock, Principal Investigator of TCF–001 TRACK, co-authored an article in the CA Cancer Clinical Journal about the opportunities and challenges of tissue-agnostic therapy development and the current landscape of approved therapies and targets.
 The future of cancer therapy has arrived, transcending the boundaries of histology and location to provide personalized, biomarker-driven treatments that hold the promise of more effective and less toxic interventions in our battle against cancer.
The future of cancer therapy has arrived, transcending the boundaries of histology and location to provide personalized, biomarker-driven treatments that hold the promise of more effective and less toxic interventions in our battle against cancer.Razelle Kurzrock, MD
Associate Director, Clinical Research, Medical College, Wisconsin
Chief Medical Officer, Worldwide Innovate Network (WIN) for Precision Medicine
Principal Investigator of TCF–001 TRACK
Value of tissue-agnostic therapies for individuals with rare cancers
Tissue-agnostic therapies are a breakthrough in cancer treatment, changing the way tumors can be treated. Instead of basing treatment on a person’s cancer type or its location in the body, treatments are chosen for their ability to target (or attack) specific genetic abnormalities in the cancer cells.
This is especially promising for patients with rare cancers that are understudied or otherwise have limited effective treatments. Tissue-agnostic therapies open the door to more personalized treatments and allow more patients to benefit from therapies that target the genomic drivers of their cancer.
Watch Dr. Subbiah’s remarks on tissue-agnostic therapies at TCF’s Think Tank on Advancing Precision Medicine in Rare Cancers.

Patients cannot wait for trials to be created in every type of cancer. By eliminating the restriction of tumor location and type, tissue-agnostic therapies are revolutionizing our concept of personalized medicine.
Vivek Subbiah, MD
Chief of Early-Phase Drug Development, Sarah Cannon Research Institute
Co-Principal Investigator of TCF–001 TRACK
An era of innovation in targeted therapies
Cancer science has advanced greatly. Researchers now have a better understanding of how different cancers form and grow. As more is learned about the genetic and molecular basis of cancer, treatments are becoming more personalized.
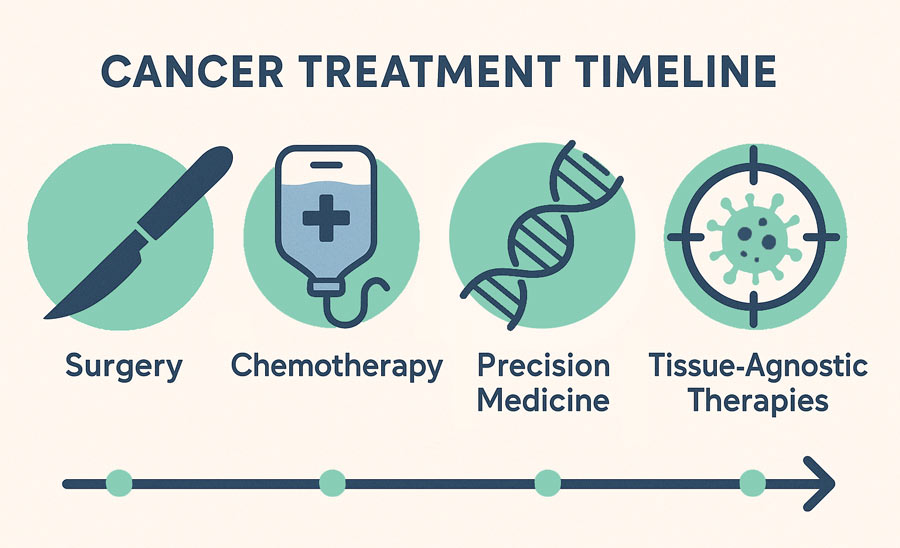
Surgery and chemotherapy were previously the most commonly used treatments for cancer. Now, oncologists have many more options, including precision medicine approaches such as tissue-agnostic therapies. Biomarker testing has revealed that many types of cancers share genetic abnormalities. And research projects like the Cancer Genome Atlas have taught us that understanding genetic abnormalities can be the key to finding effective treatments.
This knowledge, together with efficiencies in how these drugs are reviewed and approved by the FDA, has sparked an era of innovation in tissue-agnostic therapies.
Understanding the connection between biomarker testing and tissue-agnostic therapy
Biomarker testing — also known as molecular profiling, comprehensive genomic profiling, or next-generation sequencing — is essential for any rare cancer patient. It can be performed on blood or tissue. It is important to get a test that looks at multiple genes at one time. Many next-generation sequencing tests will look at several hundred genes. Because each of the tissue-agnostic molecular markers is rare, testing tissue for one marker at a time is not a practical or efficient use of time or a patient’s tumor tissue.
Biomarker testing helps a person with cancer learn if genetic alterations are present in their tumor. This allows for the consideration of tissue-agnostic therapy options targeting those alterations, including approved therapies and those being studied in clinical trials.
Example: Cholangiocarcinoma
A person with cholangiocarcinoma — a rare cancer of the bile ducts — gets biomarker testing after their diagnosis, and the test results show that their tumor has a genetic alteration called a Neurotrophic Tyrosine Receptor Kinase (NTRK) fusion. Using this information, the oncologist* can prescribe any one of three drugs that suppress NTRK and have tissue-agnostic approval from the U.S. Food and Drug Administration (FDA). This means that a patient with any solid tumor that has an NTRK fusion can receive that therapy. Because that patient had biomarker testing, some of these potent therapies became available to them.
*If you plan to get biomarker testing, seek out an oncologist with expertise in molecular-driven therapies.
CA A Cancer J Clinicians, Volume: 74, Issue: 5, Pages: 433-452, First published: 30 May 2024, DOI: (10.3322/caac.21844
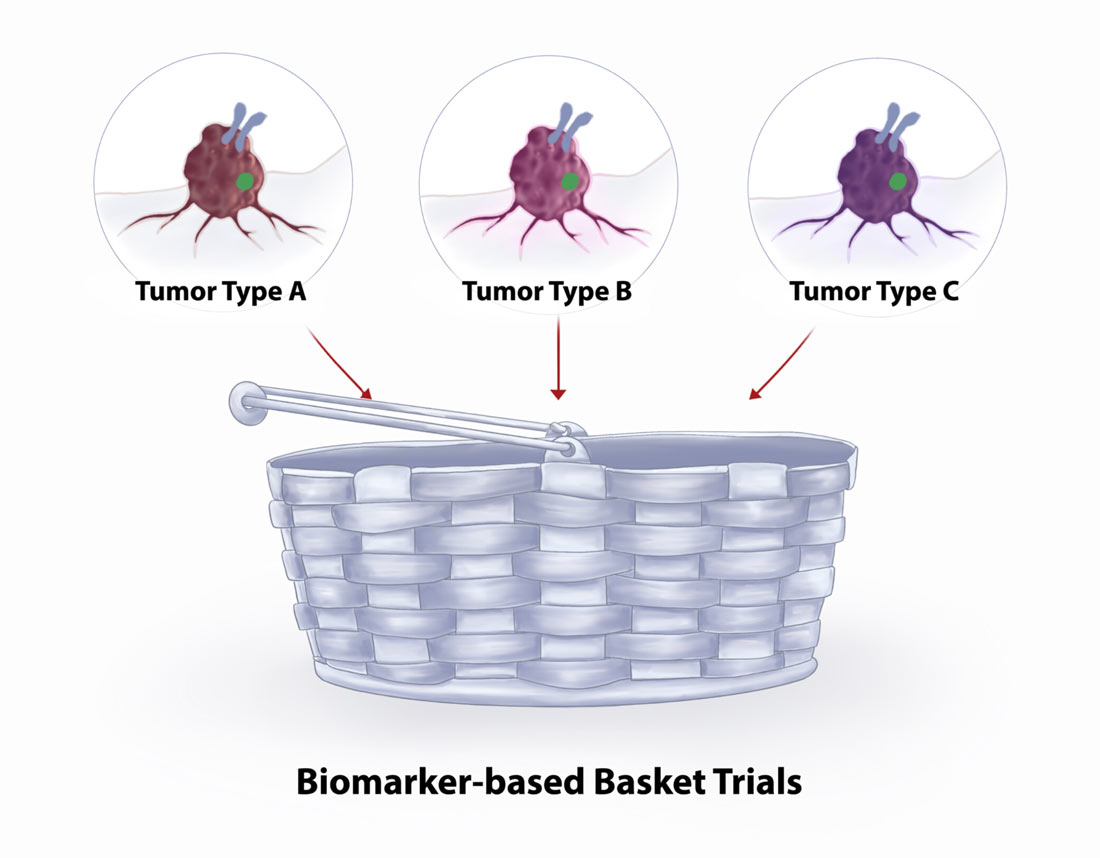
Basket trials
If you learn through biomarker testing that your cancer has genetic mutations for which there are no FDA-approved treatments, ask your doctor if you are eligible for any clinical trials. One type of clinical trial that you may specifically want to ask about is a basket trial, which tests a new drug or therapy in patients with different cancers that share the same genetic mutation. Basket trials can provide access to promising therapies that may be more likely to be effective against your rare cancer. They have the potential to lead to FDA-approved therapies, especially for rare cancers.
Why consider tissue-agnostic therapy?
- Can potentially be used in any type of cancer
- Leverages the results of biomarker testing to provide more precise therapies to patients with specific genetic alterations
- Ability to treat rare cancers that have been less frequently studied or associated with FDA-approved drugs.
US Food and Drug Administration (FDA)-approved tissue-agnostic therapies
In 2017, the FDA granted its first tissue-agnostic drug approval. Like other cancer drugs, tissue-agnostic therapies are reviewed by the FDA for safety and effectiveness. But, their development requires additional scientific considerations. These may include differences in clinical trial design and review and approval processes.
For example, tissue-agnostic therapies are not currently covered as a group by existing NCCN clinical practice guidelines, which creates barriers to awareness for clinicians about the most appropriate care to offer. This is something TargetCancer Foundation is actively working to change.
As of 2025, there are ten FDA-approved tissue-agnostic therapies, with many more in development. A list of FDA-approved tissue-agnostic therapies is available on the American Cancer Society website.
Benefits of tissue-agnostic drug development
As the number of tissue-agnostic drug approvals grows, and the pathway for development becomes clearer, pharmaceutical companies are focusing more on these types of treatments.
Some of the benefits of tissue-agnostic drug development include:
- Improved patient access — People with uncommon or advanced cancers with specific mutations may be able to access effective treatments that were previously unavailable.
- Accelerated development timelines because drug developers do not need to conduct separate clinical trials for each tumor type.
- Streamlined review and approval process.
- Promising results in treating various cancers driven by a shared biomarker.
- Allows for a better understanding of molecular pathways and cancer growth mechanisms, propelling precision medicine forward.
Tissue-agnostic approvals are especially important for people with rare cancers. It can be very difficult to do a clinical trial for a rare subset (that is, a biomarker-defined subset) of an already rare cancer. Tissue-agnostic approvals ensure that these targeted, high-response-rate drugs are available to patients with rare cancers without needing separate trials for each cancer type.
Rare Cancer Industry Roundtable
In 2023, TargetCancer Foundation convened its first Rare Cancer Industry Roundtable. This event united leaders in rare cancer and tissue-agnostic drug development to identify opportunities for cross-sector collaboration. From the conversations at this event, TargetCancer Foundation developed new programs to bring greater access to tissue-agnostic therapies for both patients and clinicians.
TargetCancer Foundation’s 2023 Industry Roundtable
Questions to ask your doctor about tissue-agnostic therapy for rare cancers
If you are considering whether or not a tissue-agnostic therapy is right for you, you should ask your care team questions about what to expect. Possible questions may include:
- Do the results of my biomarker testing show any genetic alterations that can be targeted by an existing tissue-agnostic therapy?
- If so, how would taking this tissue-agnostic therapy compare to not receiving treatment or taking a different drug or treatment?
- What are the expected side effects of this tissue-agnostic therapy?
- How long will I receive this therapy? Where will I need to go to get this therapy? What is the dosing frequency?
- How will this therapy be given (e.g., pill, injection, infusion)?
- How will I know if the therapy is working?
- What are the names of the drug(s) I will be taking?
- How do I find out if my insurance covers tissue-agnostic therapies?
- Have you treated other patients with tissue-agnostic therapies?
- If so, what kinds of results have you seen?
- If not, can you recommend a provider who has experience with these types of therapies?
Additional resources
- Tumor-Agnostic Drugs (Cancer.org)
- Tissue-Agnostic Drug Development in Oncology (FDA.gov)
- The Evolving Landscape of Tissue-Agnostic Therapies in Precision Oncology (ACS Journals)
- New Tissue-Agnostic Treatment Options Emerge (ONClive.com)