A National Precision Genomics Clinical Trial for Rare Cancers
Developed and launched by TargetCancer Foundation, TCF-001 TRACK is a fully remote clinical trial enrolling 400 patients with rare cancers or cancers of unknown primary.
Enrolled patients and their physicians receive biomarker (genomic) testing of both tissue and blood as well as interpretation and treatment recommendations from an expert panel of rare cancer clinicians and scientists. Simultaneously, TRACK generates critical genomic data to drive a better understanding of often overlooked rare cancers.
TRACK Data Presented at the 2024 ASCO Annual Meeting
TargetCancer Foundation was honored to have its abstract selected among thousands of submissions for a poster presentation at the 2024 ASCO Annual Meeting in Chicago. This represented the first public presentation of TRACK data, and provided an opportunity to showcase TRACK for the over 44,000 meeting attendees from around the world.
The poster was presented by TargetCancer Foundation CEO Jim Palma, as well as TRACK team members Dr. Mina Nikanjam and Dr. Jacob Adashek, and Lincoln Pasquina, PhD, from Foundation Medicine.
TCF-001 TRACK's Impact
Enrolled
patients
From
states
With
different types of rare cancers
Decentralized, Fully-Remote Consenting Process
Through a remote consenting process, patients can enroll in the TRACK study from their homes, without traveling to a clinical trial site.
TCF-001 TRACK Rare Cancer Day Webinar
A discussion on precision medicine in rare cancers
TRACK — which stands for Target Rare Cancer Knowledge — was developed completely in response to what we hear from patients and doctors every day. There are many challenges and difficulties that people are facing that are very consistent no matter the type of rare cancer. So we tried to take those experiences and craft a study that simultaneously impacts the patient experience while driving research forward.
Jim Palma
CEO, TargetCancer Foundation
Virtual Molecular Tumor Board (VMTB)
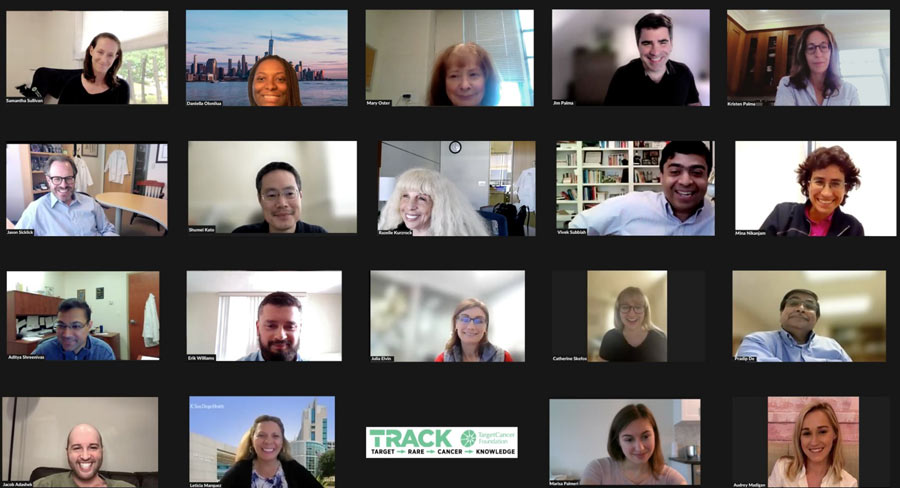
Virtual Molecular Tumor Board
The TRACK VMTB unites a brain trust of medical oncologists, surgeons, pathologists, genetic counselors, and others who specialize in rare cancers and are experts in interpreting the biomarker testing reports that a TRACK patient receives.
The group meets weekly and reviews each patient’s case for an in-depth discussion of their medical history and individual biomarker testing reports to offer personalized treatment recommendations for difficult-to-treat cancers.
Access to the VMTB allows patients anywhere in the country to have experienced physicians and experts in rare tumors review their case, which is especially important for patients treated outside of major academic centers, who may not have access to specialists in their rare cancer.
Questions? Read our FAQ.
Meet the TRACK Investigators and Study Team.
Learn More About Track
Research Collaborator

More About TCF-001 TRACK

“Home-run trials for rare cancers” by Razelle Kurzrock, MD and Jacob Adashek, DO – Read Article




